SELECT: A phase 3, multicenter, randomized, double-blind, placebo-controlled trial in patients with locally recurrent or metastatic RAI-R DTC1
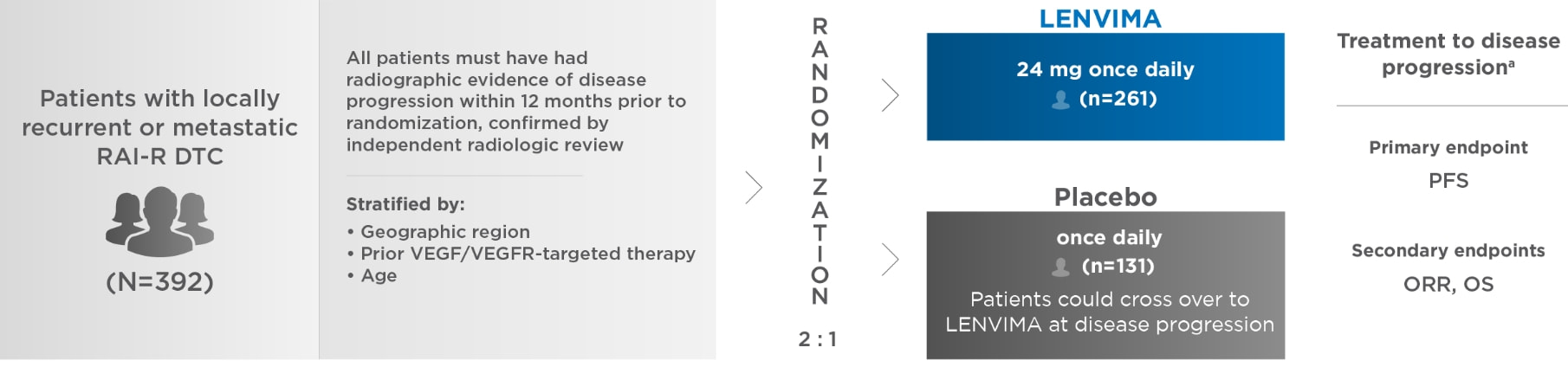
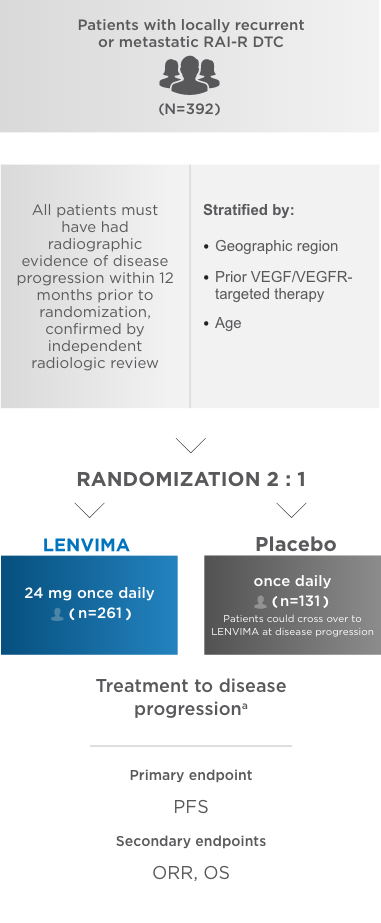
SELECT=Study of (E7080) LEnvatinib in Differentiated Cancer of the Thyroid; RAI-R=radioactive iodine-refractory; DTC=differentiated thyroid cancer; VEGF=vascular endothelial growth factor; VEGFR=vascular endothelial growth factor receptor; PFS=progression-free survival; ORR=objective response rate; OS=overall survival.
aDetermined by blinded, independent radiologic review using Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1.
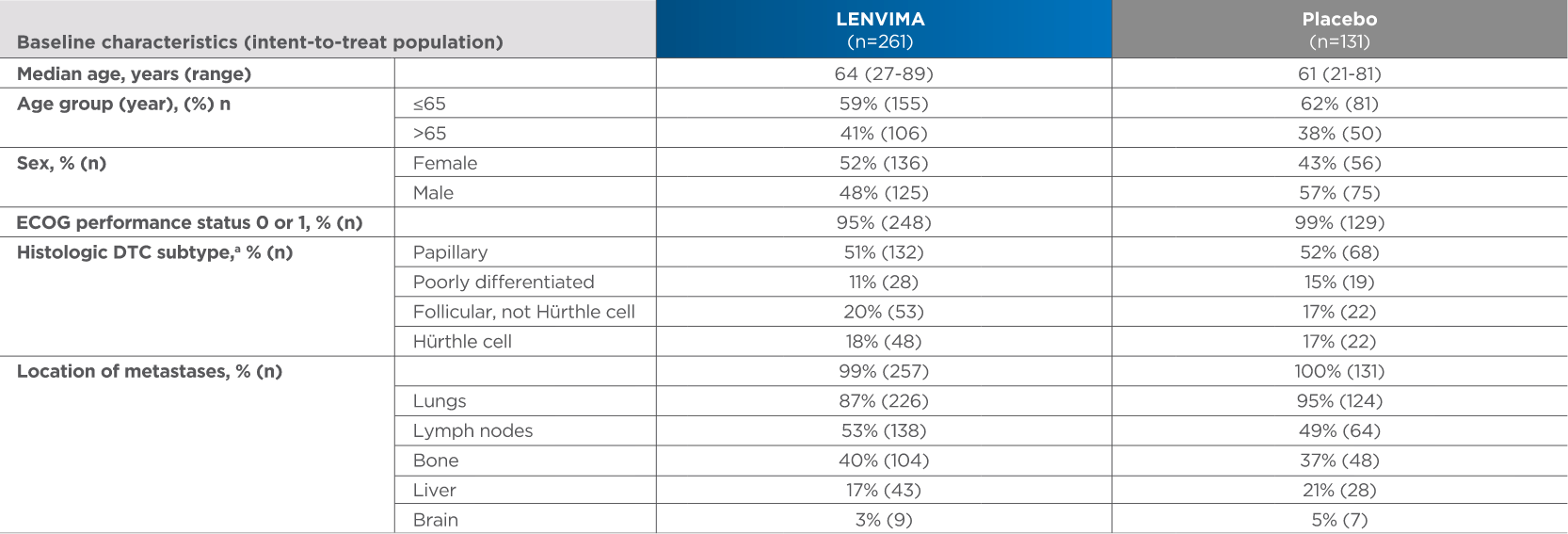
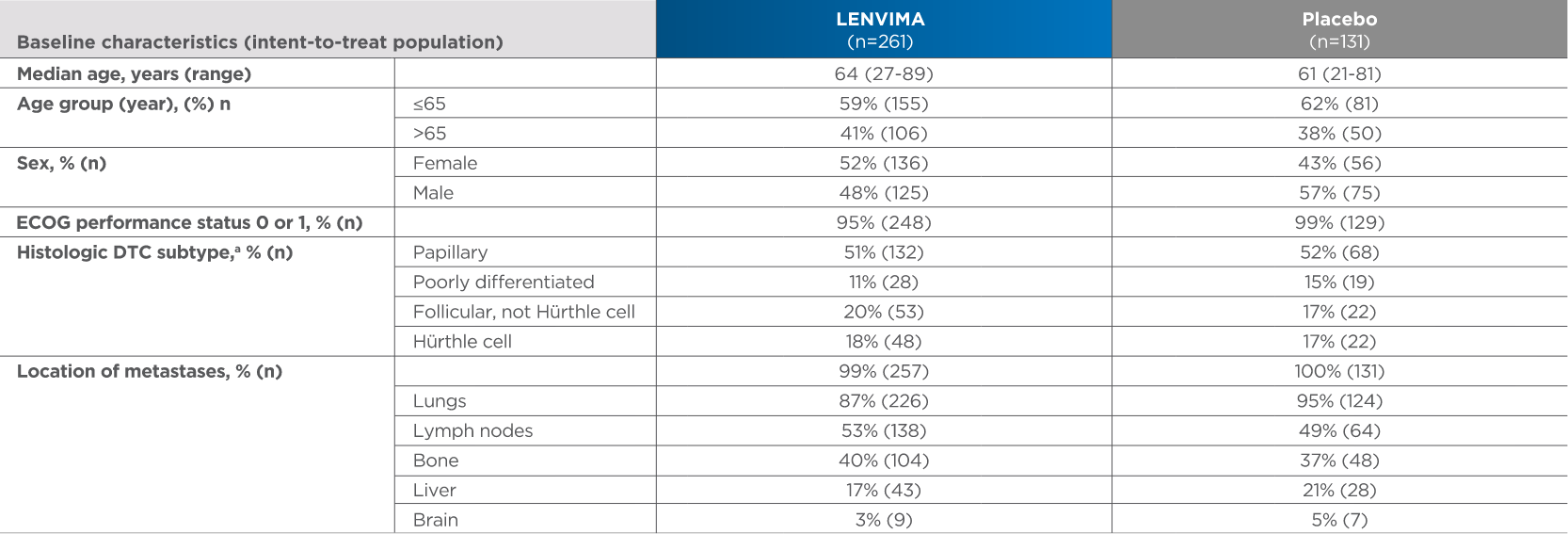
Baseline patient characteristics2,3
-
4 out of 10 patients were >65 years old
The median cumulative RAI activity administered prior to study entry was 350 mCi (12.95 GBq).1
ECOG=Eastern Cooperative Oncology Group; RAl=radioactive iodine; mCi=millicurie; GBq=gigabecquerel.
aHistologic findings were determined from investigators’ reports.
