Recommended dose and dose modifications1,a
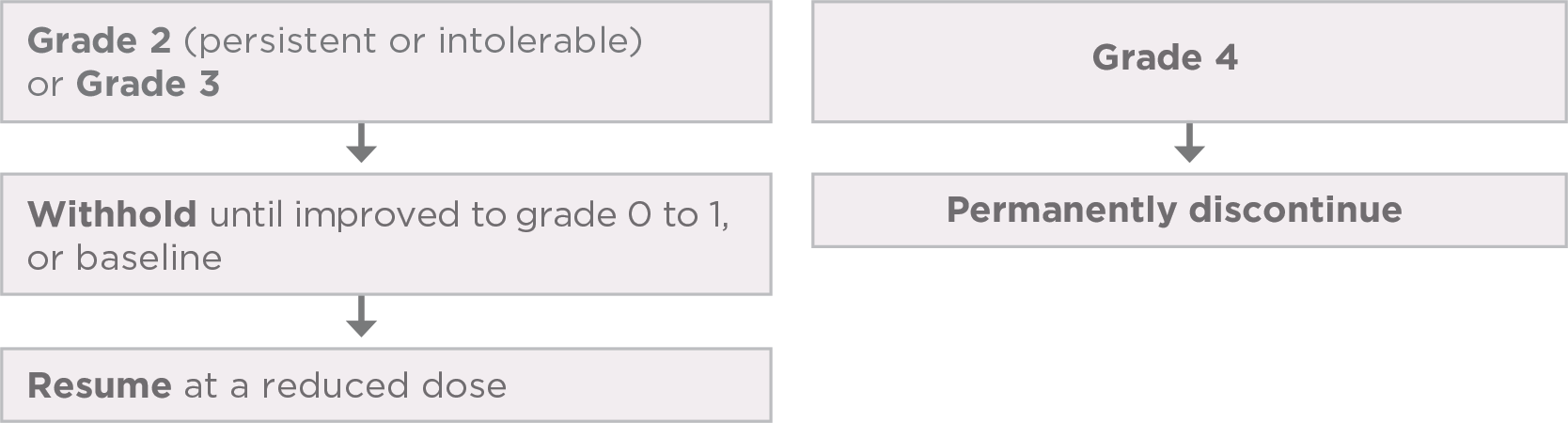
Interrupt, reduce, and/or discontinue LENVIMA based on the type and/or severity (grade) of the adverse reaction
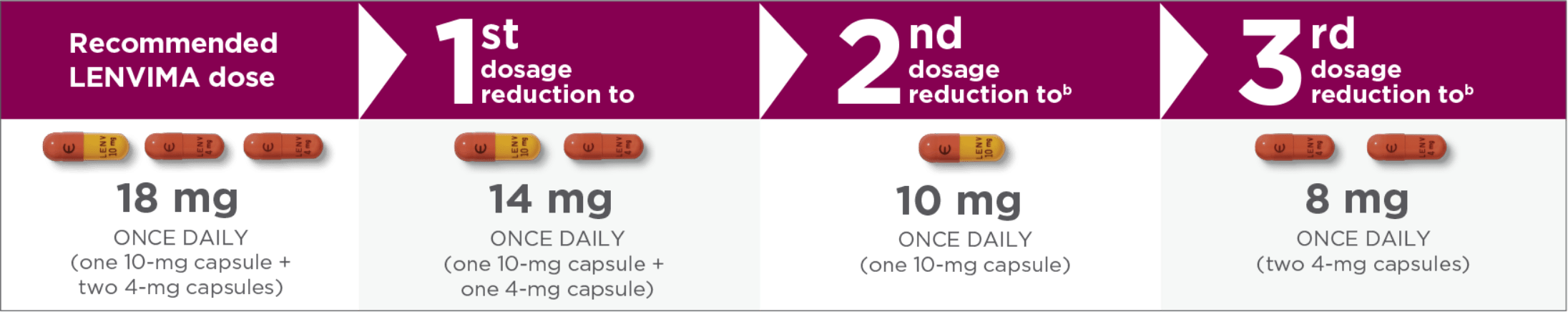
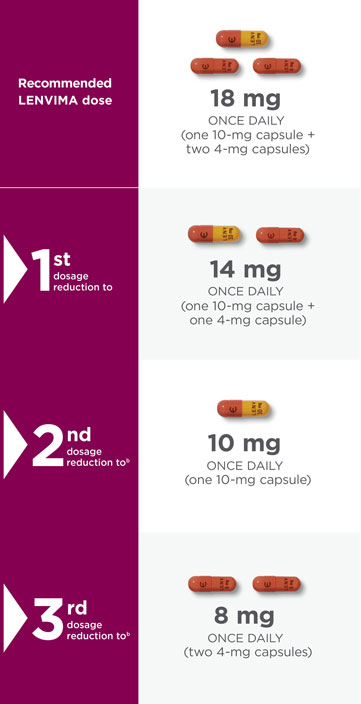
Capsules pictured are not actual size.
Review the full Prescribing Information for everolimus for recommended dose modifications1,c

Proactively plan to manage nausea, vomiting, or diarrhea with concomitant medications as necessary prior to interruption or dose reduction of LENVIMA.
- In Study 205, ARs led to dose reductions and interruption in 89% of patients receiving LENVIMA + everolimus1
- Treatment discontinuation due to ARs occurred in 29% of patients receiving LENVIMA + everolimus1
AR=adverse reaction.
- aReduce dose in succession based on the previous dose level (18 mg, 14 mg, 10 mg, or 8 mg per day).
- bRefers to the same or a different adverse reaction that requires dose modification.
- cRefer to everolimus Prescribing Information for additional dose modification information.
For the management of specific adverse reactions, please see the Adverse Reaction Management section below.
AR management
Help manage ARs with PI-guided strategies
Click on the bar of each AR for PI-guided strategies. For the complete list of ARs, see full Prescribing Information.
-
Decreased appetite


PI-guided strategies to help manage decreased appetite1
-
Decreased weight


PI-guided strategies to help manage decreased weight1

CTCAE v4.0 does not define grade 4 decreased weight. Permanently discontinue for grade 4 adverse reactions.
-
Diarrhea

PI-guided strategies to help manage diarrhea1
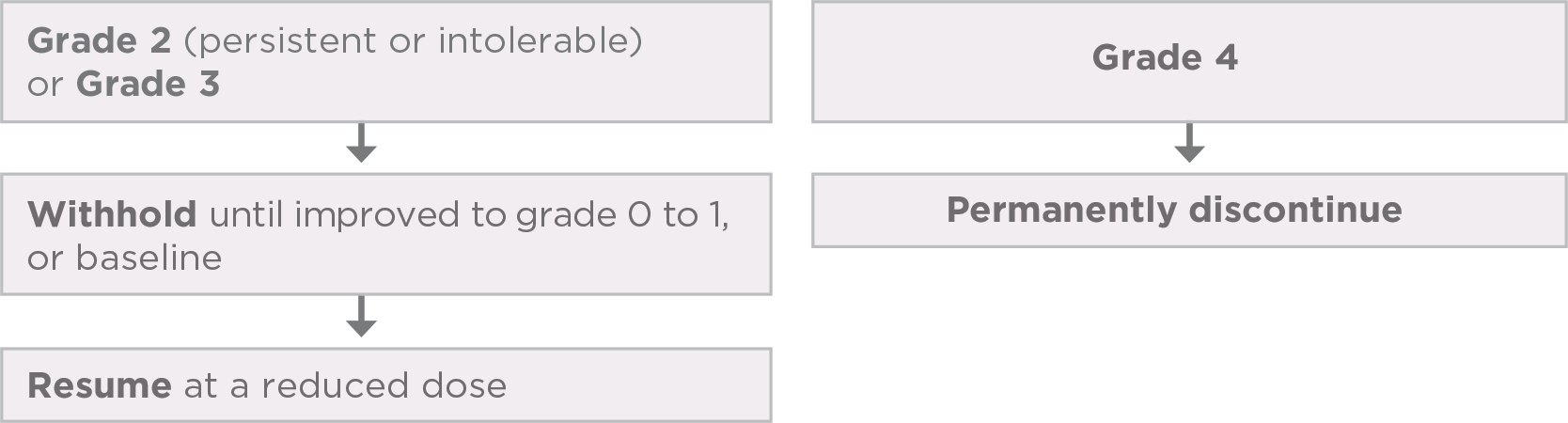
Promptly initiate management of diarrhea. Withhold and resume at a reduced dose upon recovery, or permanently discontinue LENVIMA based on severity.1
-
Fatigue


PI-guided strategies to help manage fatigue1

CTCAE v4.0 does not define grade 4 fatigue. Permanently discontinue for grade 4 adverse reactions.
-
Hypertension


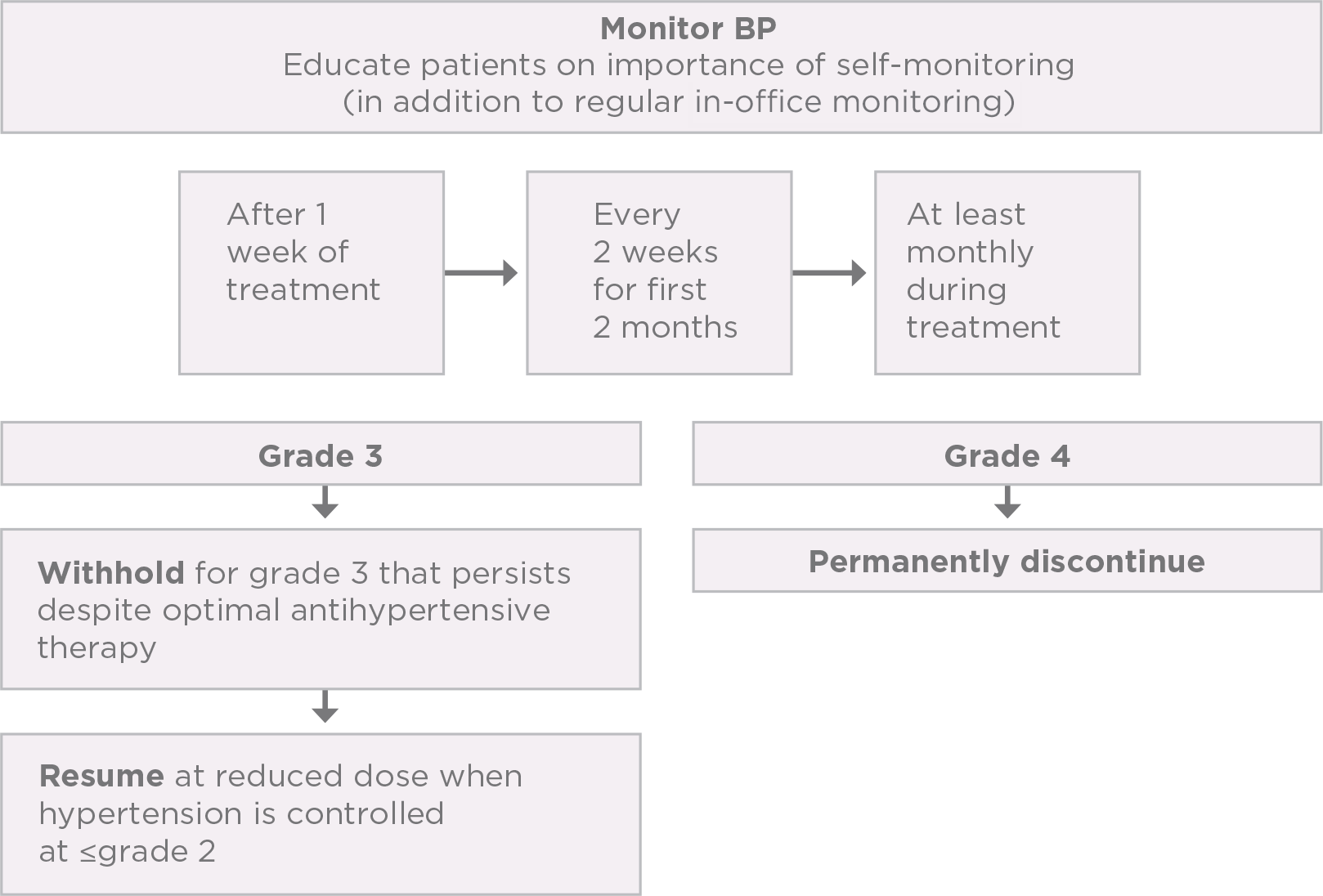
PI-guided strategies to help manage hypertension1
Control blood pressure prior to initiating treatment
-
Nausea
PI-guided strategies to help manage nausea1

CTCAE v4.0 does not define grade 4 nausea. Permanently discontinue for grade 4 adverse reactions.
-
Proteinuria


PI-guided strategies to help manage proteinuria1
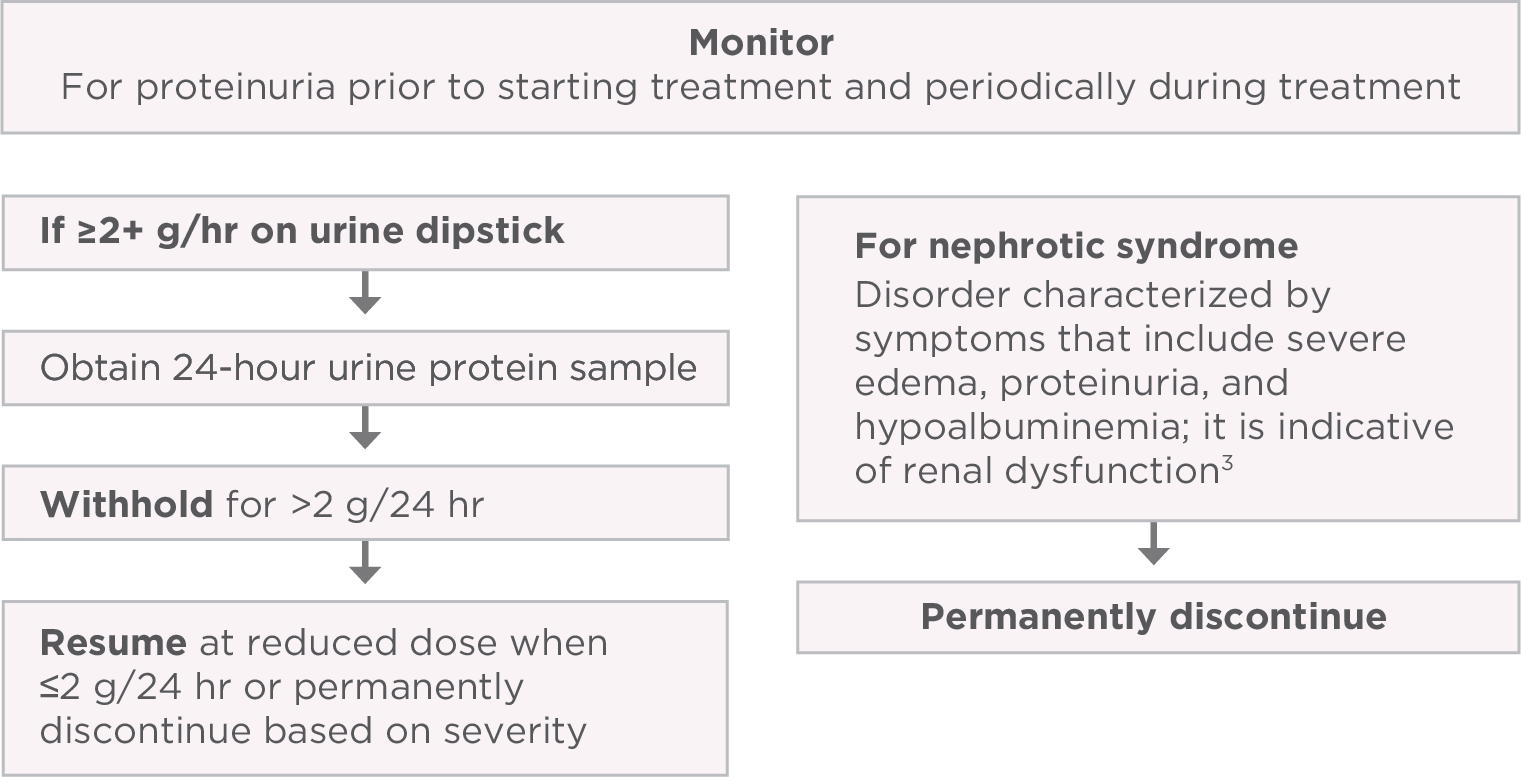
AR=adverse reaction; PI=Prescribing Information; CTCAE=Common Terminology Criteria for Adverse Events; BP=blood pressure.
