NCCN recommendation
LENVIMA + everolimus
The only 2L TKI-mTOR inhibitor combination approved for adults with aRCC following prior anti-angiogenic therapy1,2

Lenvatinib (LENVIMA) + everolimus has a National Comprehensive Cancer Network® (NCCN®) category 2A other recommended regimen as a subsequent therapy option for patients with relapse or stage IV clear cell RCC*†
NCCN=National Comprehensive Cancer Network® (NCCN®).
*Category 2A: Based upon lower-level evidence, there is uniform NCCN consensus that the intervention is appropriate.
†Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Kidney Cancer V.4.2023. © National Comprehensive Cancer Network, Inc. 2023. All rights reserved. Accessed January 18, 2023. To view the most recent and complete version of the guideline, go online to NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use, or application and disclaims any responsibility for their application or use in any way.
MAJOR EFFICACY OUTCOME
14.6 months median PFS with the combination1
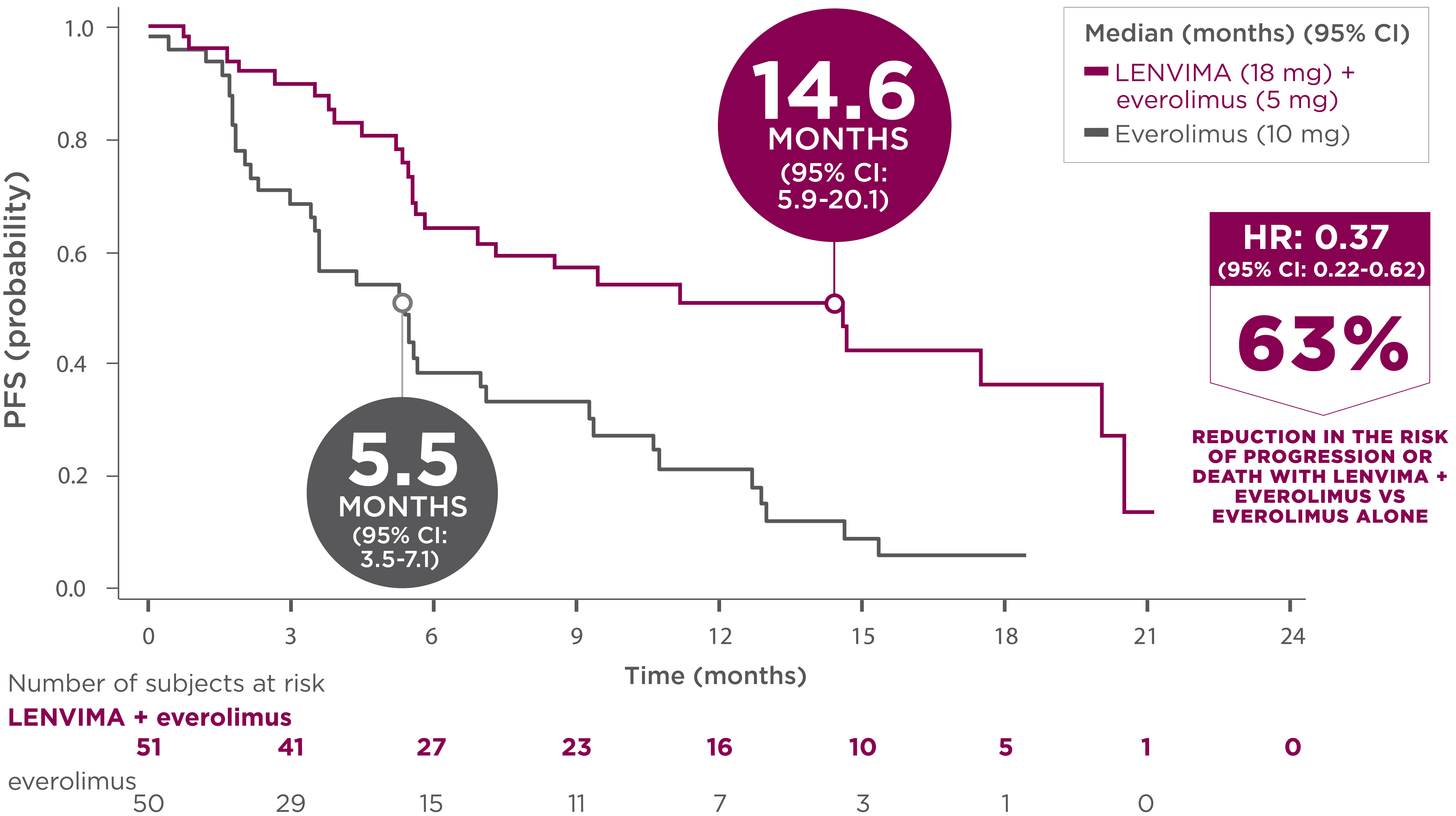
-
26 events (51%) occurred in the LENVIMA + everolimus arm vs 37 events (74%) in the everolimus arm
- 21 patients (41%) who received LENVIMA + everolimus progressed vs 35 patients (70%) who received everolimus
- Death occurred in 5 patients (10%) who received LENVIMA + everolimus vs 2 patients (4%) who received everolimus
- The treatment effect of LENVIMA + everolimus on PFS was supported by a retrospective, independent review of radiographs with an observed HR of 0.43 (95% CI: 0.24-0.75) compared with the everolimus arm
TKI=tyrosine kinase inhibitor; mTOR=mammalian target of rapamycin; aRCC=advanced renal cell carcinoma; PFS=progression-free survival; OS=overall survival; ORR=objective response rate; 2L=second line.
OTHER EFFICACY OUTCOME
Greater than 2 years median OS with the combination1
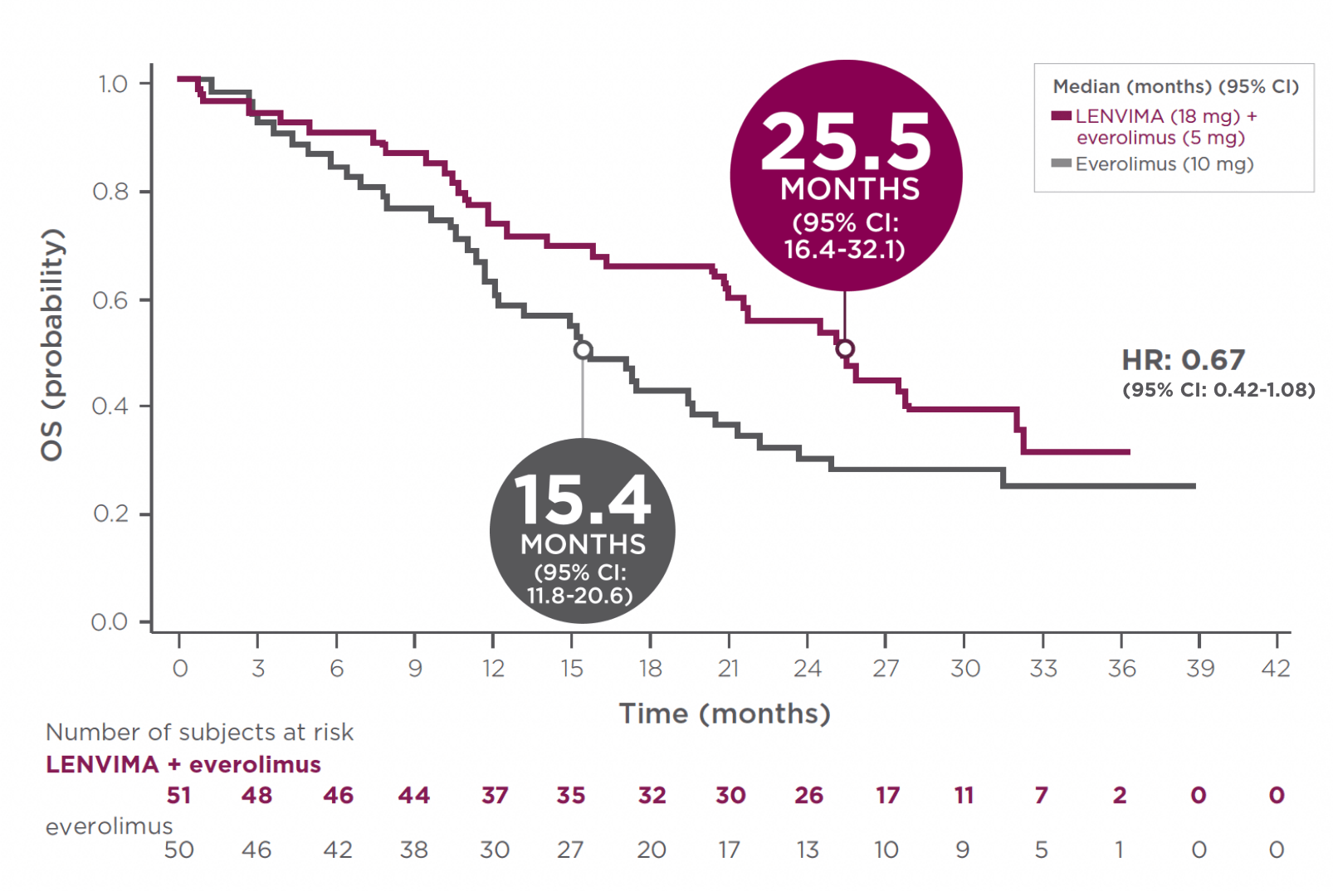
- At the time of analysis, 63% of deaths (32 patients) had occurred in the LENVIMA + everolimus arm and 74% of deaths (37 patients) had occurred in the everolimus arm
OS=overall survival; HR=hazard ratio.
OTHER EFFICACY OUTCOME
37% response rate with the combination1

- aEvaluated according to Response Evaluation Criteria In Solid Tumors (RECIST) 1.1. Objective response rate (ORR)=sum of CR and PR.1
6x more patients responded to LENVIMA + everolimus1
- The ORR was supported by a retrospective, blinded, independent radiologic review of scans1,3
- Tumor assessments were based on RECIST v1.1 criteria for progression but only confirmed responses are included for ORR1
2L=second line; ORR=objective response rate; PR=partial response; CR=complete response.
