Adverse reactions observed in the REFLECT trial
Most common serious ARs (≥2%) in the LENVIMA arm of the REFLECT trial1
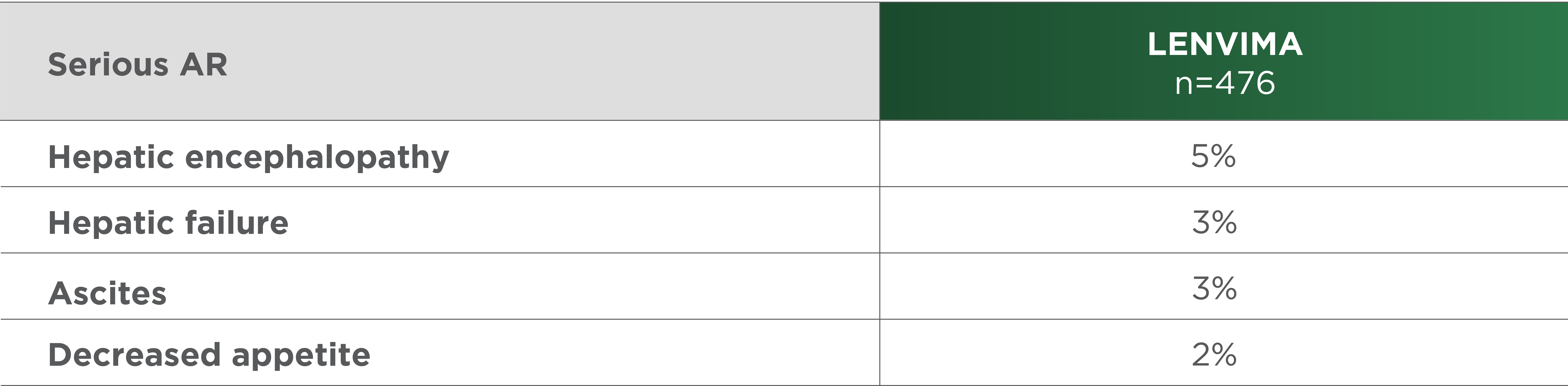
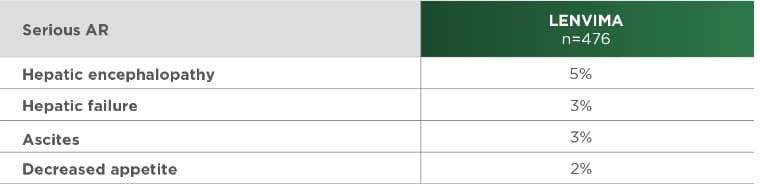
REFLECT=A Multicenter, Randomized, Open-label, Phase 3 Trial to Compare the EFficacy and Safety of LEnvatinib (E7080) Versus Sorafenib in First-Line Treatment of Subjects With UnreseCtable HepaTocellular Carcinoma; AR=adverse reaction.
REFLECT was not designed to demonstrate a statistically significant reduction in AR rates for LENVIMA vs sorafenib.1
Most common grade 3-4 ARs (≥5%) in either arm1
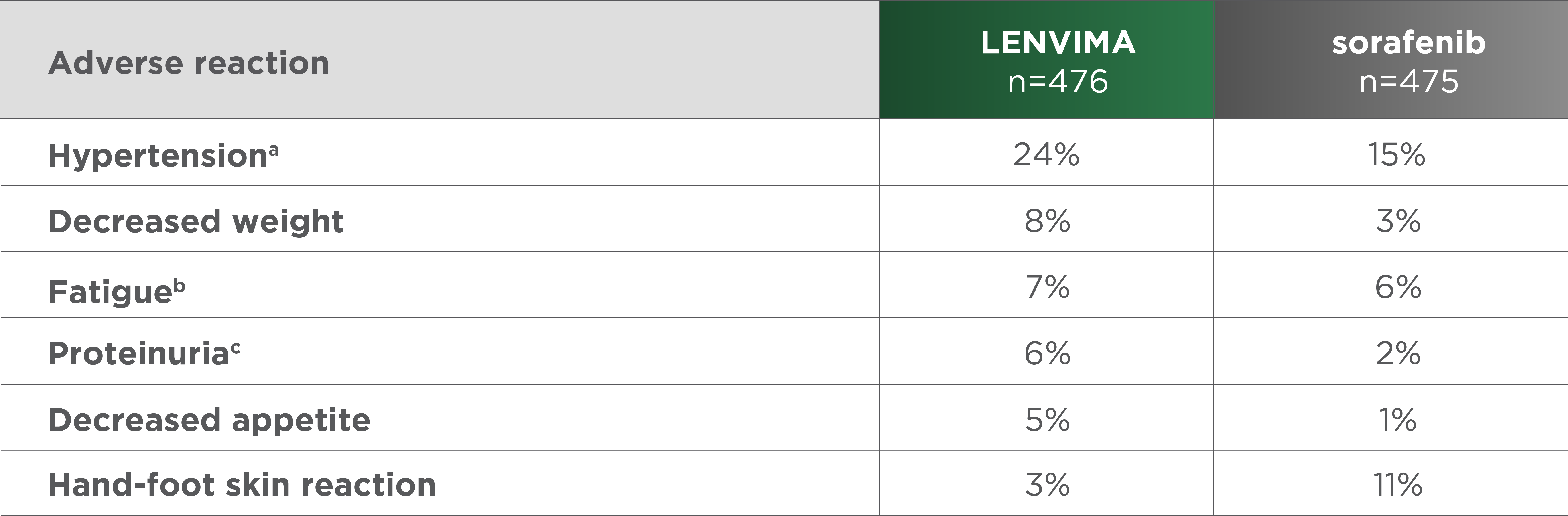
No grade 4 hypertension, proteinuria, decreased appetite, or diarrhea were reported in REFLECT.2
REFLECT was not designed to demonstrate a statistically significant reduction in AR rates for LENVIMA vs sorafenib.1
REFLECT=A Multicenter, Randomized, Open-label, Phase 3 Trial to Compare the EFficacy and Safety of LEnvatinib (E7080) Versus Sorafenib in First-Line Treatment of Subjects With UnreseCtable HepaTocellular Carcinoma; AR=adverse reaction.
- aIncludes increased diastolic blood pressure, increased blood pressure, hypertension, and orthostatic hypertension.
- bIncludes asthenia, fatigue, lethargy, and malaise.
- cIncludes proteinuria, increased urine protein, and protein urine present.
ARs occurring in ≥10% of patients in the LENVIMA arm1
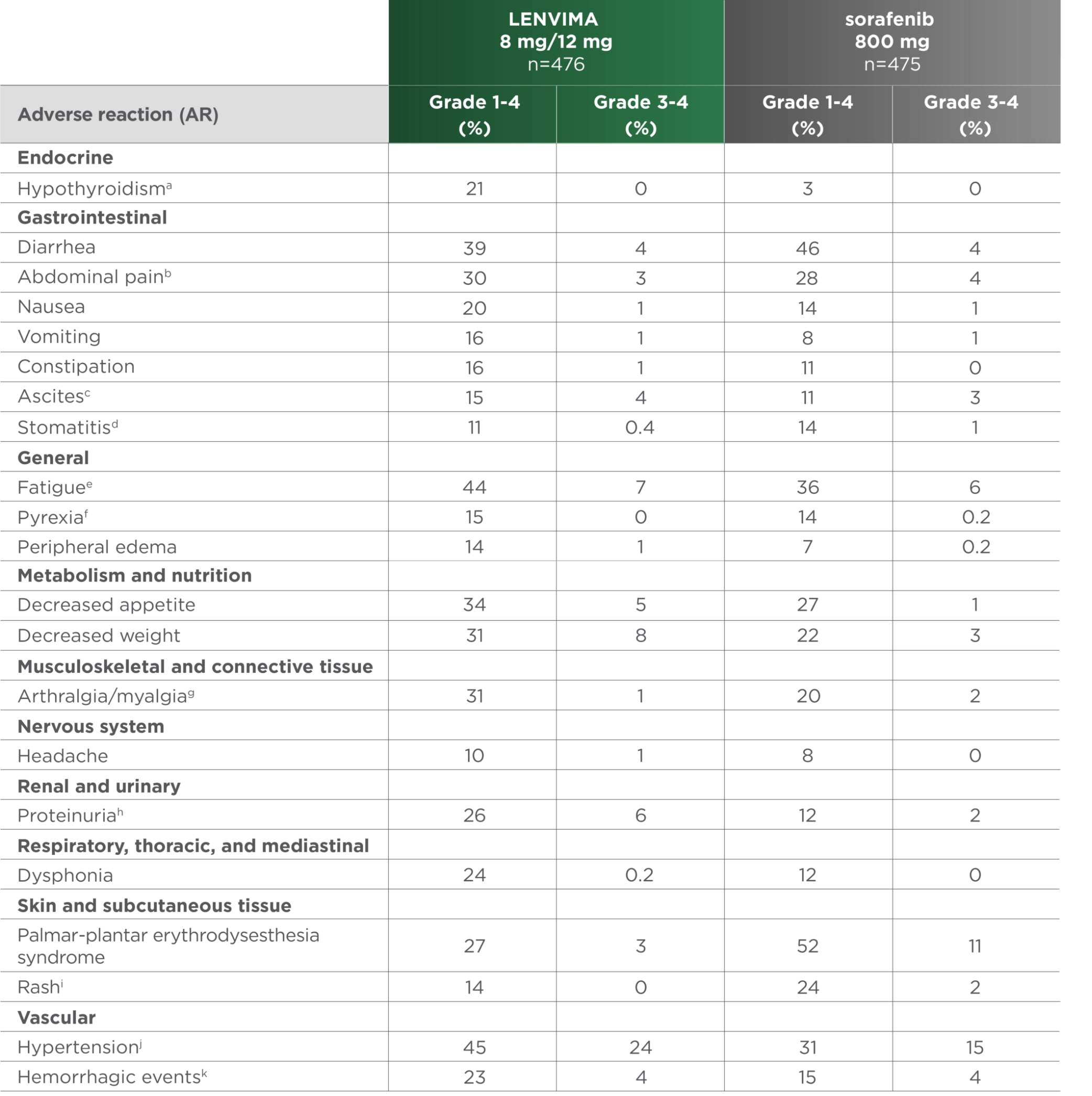
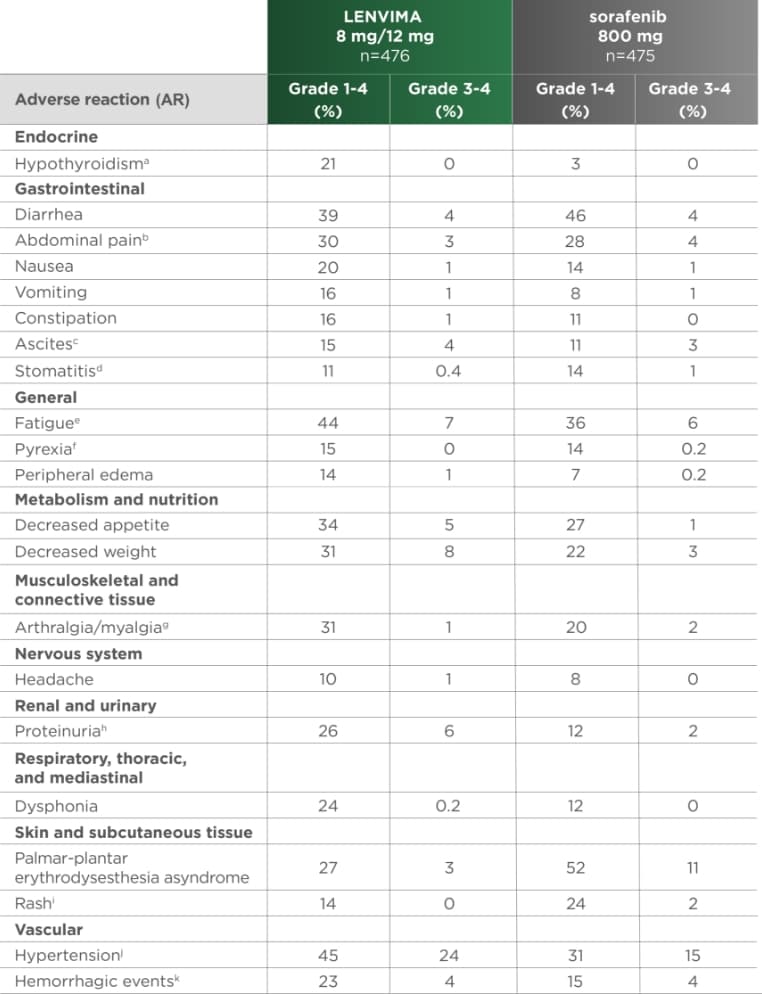
REFLECT was not designed to demonstrate a statistically significant reduction in AR rates for LENVIMA vs sorafenib.1
REFLECT=A Multicenter, Randomized, Open-Label, Phase 3 Trial to Compare the EFficacy and Safety of LEnvatinib (E7080) Versus Sorafenib in First-Line Treatment of Subjects With UnreseCtable HepaTocellular Carcinoma; HCC=hepatocellular carcinoma; AR=adverse reaction.
- aIncludes hypothyroidism and blood thyroid-stimulating hormone increased.
- bIncludes abdominal discomfort, abdominal pain, abdominal tenderness, epigastric discomfort, gastrointestinal pain, lower abdominal pain, and upper abdominal pain.
- cIncludes ascites and malignant ascites.
- dIncludes aphthous ulcer, gingival erosion, gingival ulceration, glossitis, mouth ulceration, oral mucosal blistering, and stomatitis.
- eIncludes asthenia, fatigue, lethargy, and malaise.
- fIncludes increased body temperature and pyrexia.
- gIncludes arthralgia, back pain, extremity pain, musculoskeletal chest pain, musculoskeletal discomfort, musculoskeletal pain, and myalgia.
- hIncludes proteinuria, increased urine protein, and protein urine present.
- iIncludes erythema, erythematous rash, exfoliative rash, genital rash, macular rash, maculo-papular rash, papular rash, pruritic rash, pustular rash, and rash.
- jIncludes increased diastolic blood pressure, increased blood pressure, hypertension, and orthostatic hypertension.
- kIncludes all hemorrhage terms. Hemorrhage terms that occurred in 5 or more subjects in either treatment group include: epistaxis, hematuria, gingival bleeding, hemoptysis, esophageal varices hemorrhage, hemorrhoidal hemorrhage, mouth hemorrhage, rectal hemorrhage and upper gastrointestinal hemorrhage.
Post hoc analysis of time to first onset of select ARs2
Identify points in treatment when select ARs emerged with LENVIMA in the REFLECT trial
Median weeks; AR (n=476)*
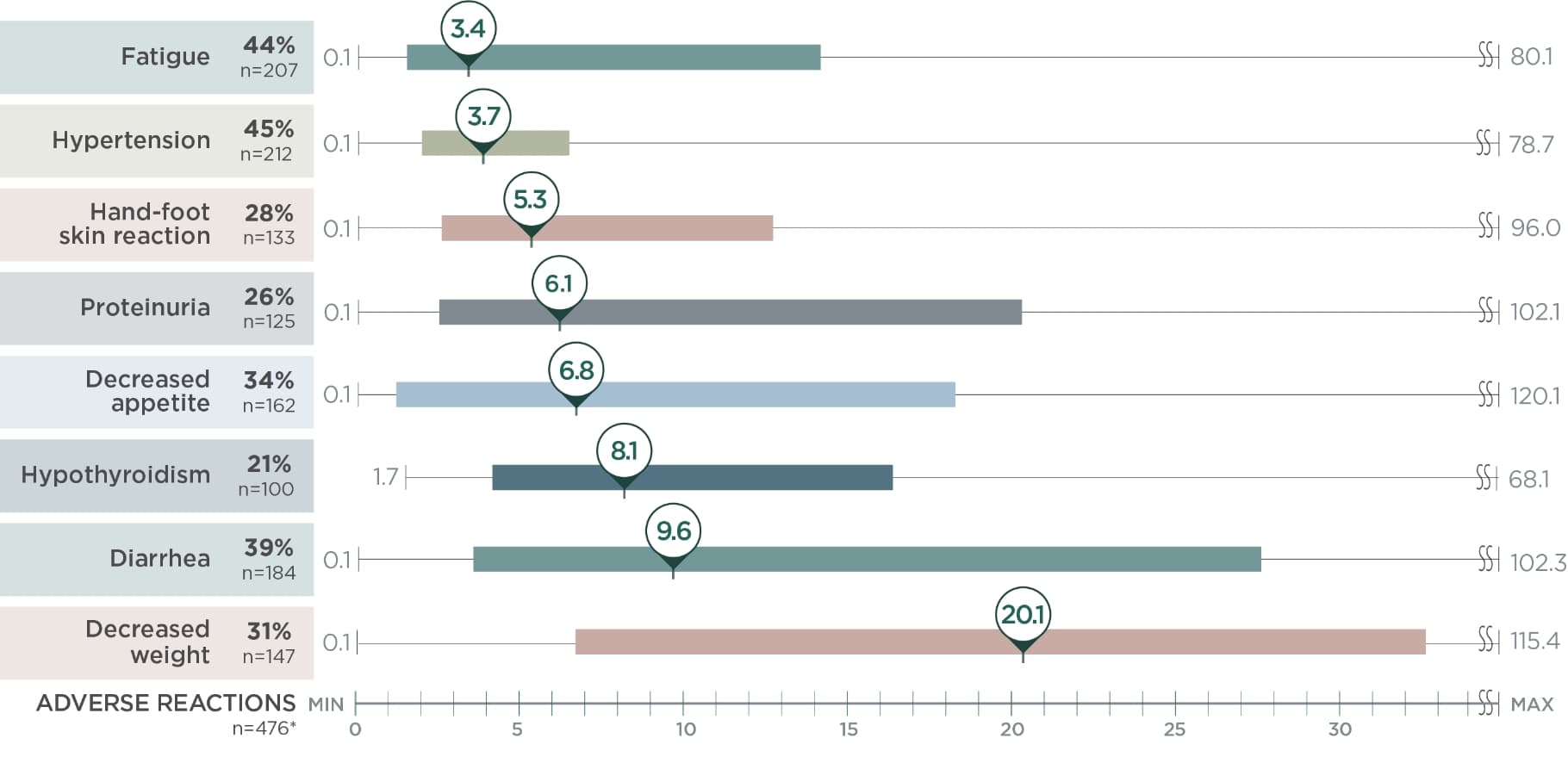
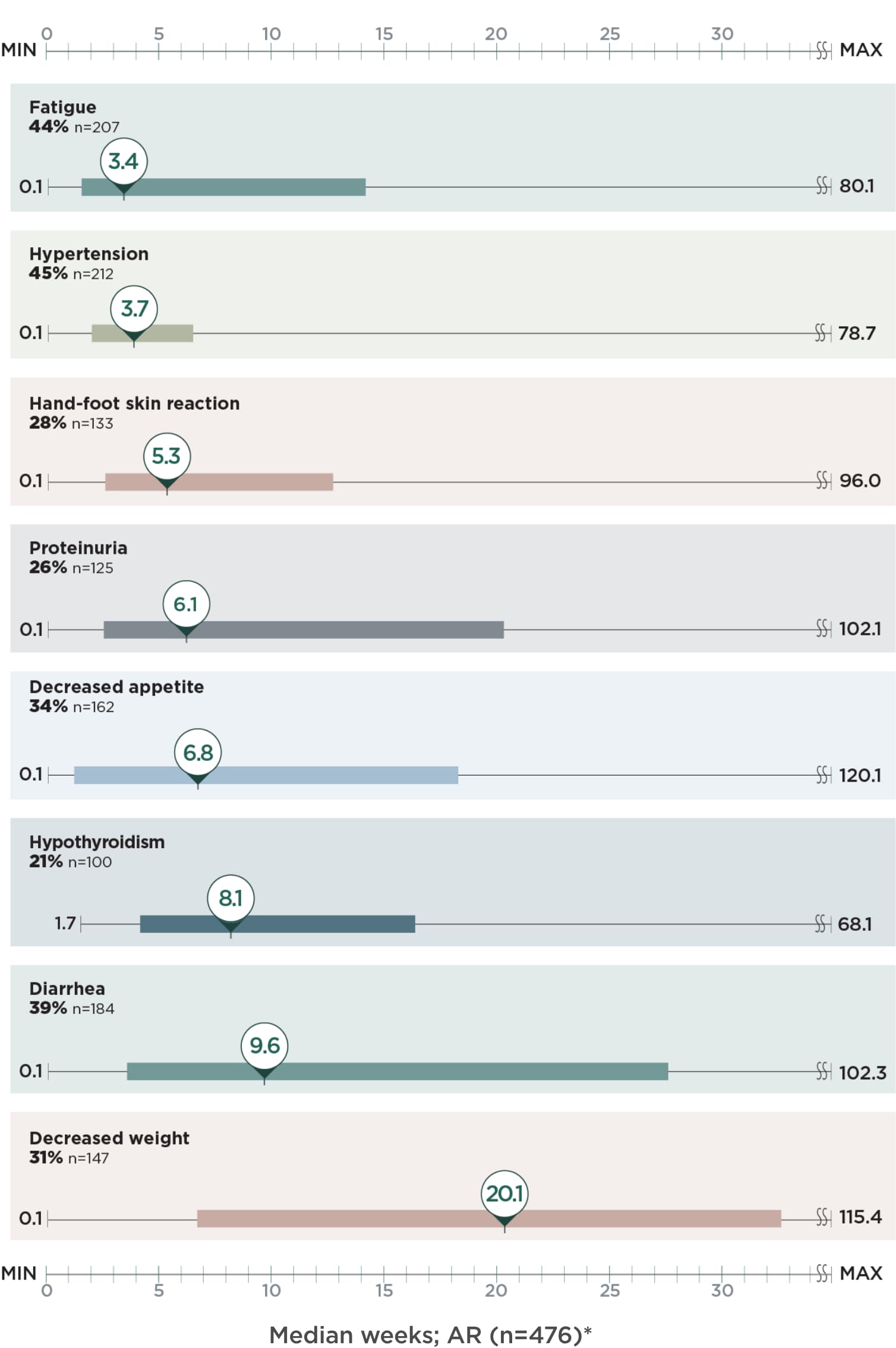
Median weeks; AR (n=476)*
- Monitor your patients for ARs throughout treatment with LENVIMA
Limitation: This is a post hoc exploratory analysis for descriptive purposes only; no conclusions can be drawn.
AR=adverse reaction.
*The bar represents the time to first onset of select ARs for the middle 50% of the patients who experienced that AR from quartile 1 to 3.
